U.S. FDA stops sale of Pelvic Mesh
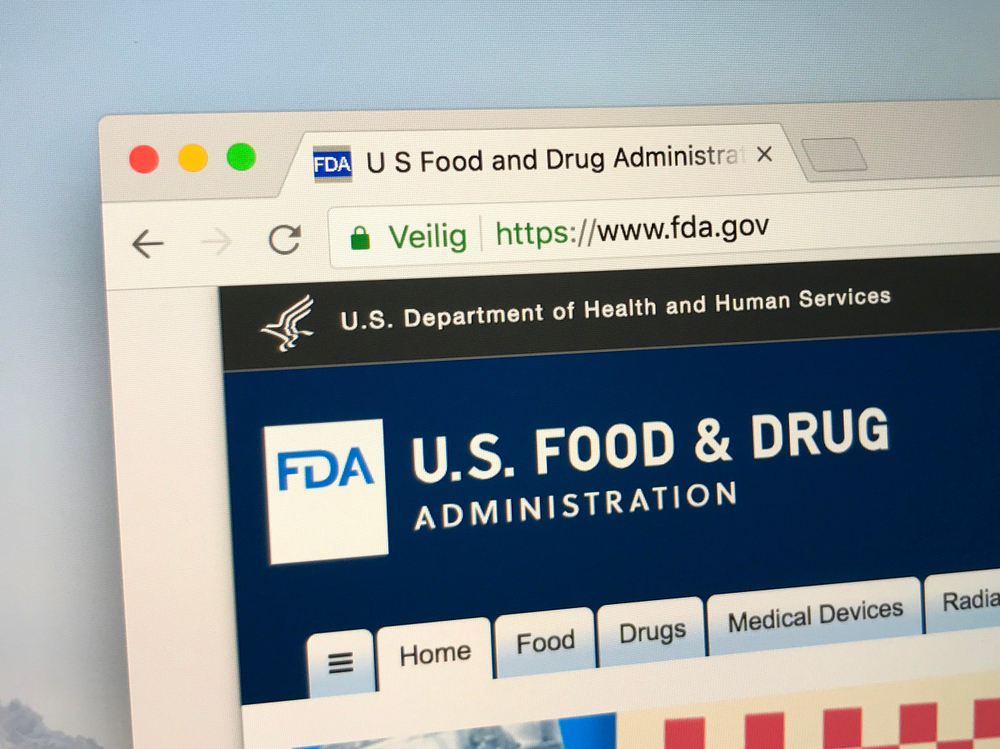
On Tuesday, U.S. Food and Drug Administration has ordered the selling and distribution of pelvic mesh.
The surgical mesh was used for the repair of pelvic organ prolapse. There were two companies that were selling this mesh.
This is the strictest action the FDA has taken regarding this synthetic product. This product was implanted in millions of women to provide strength to the pelvic muscles.
The two companies that were still selling this product were Boston Scientific and Coloplast. This decision comes at a time when there were multimillion-dollar verdicts were coming against them.
Pelvic mesh is also known as transvaginal mesh. The litigation over this product is the top ranking as far as claims filed, number of corporate defendants and settlement dollars.
Companies like Boston Scientific and Johnson & Johnson are paying $8 billion to more than 100,000 women to resolve the claims filed in court.
Women and legal advocates have been trying to persuade the FDA that the pelvic mesh is causing harm to women’s bodies. The FDA classified pelvic mesh as high risk in 2016.
The FDA asked the companies to provide evidence of safety of the product.
The FDA said that the companies, Boston Scientific and Coloplast didn’t provide enough evidence regarding the safety issues.
A spokesperson for the agency said that FDA are giving the companies 10 days to give a plan of how they are going to withdraw the product from the markets.
Shanin Specter has been fighting against such firms say that the FDA has done a good job but the job is not over. They should extend their decision and include mesh devices for treatment of some urinary conditions.
The companies are disappointed by the decision of FDA. Their market value has also dropped after the decision of Food & Drug Administration.
Photo Jarretera / Shutterstock.com